
If you have received the full trial dose or. The Oxford-AstraZeneca and Pfizer BioNTech vaccines which are available in the UK went through clinical trials here to prove their safety and efficacy.
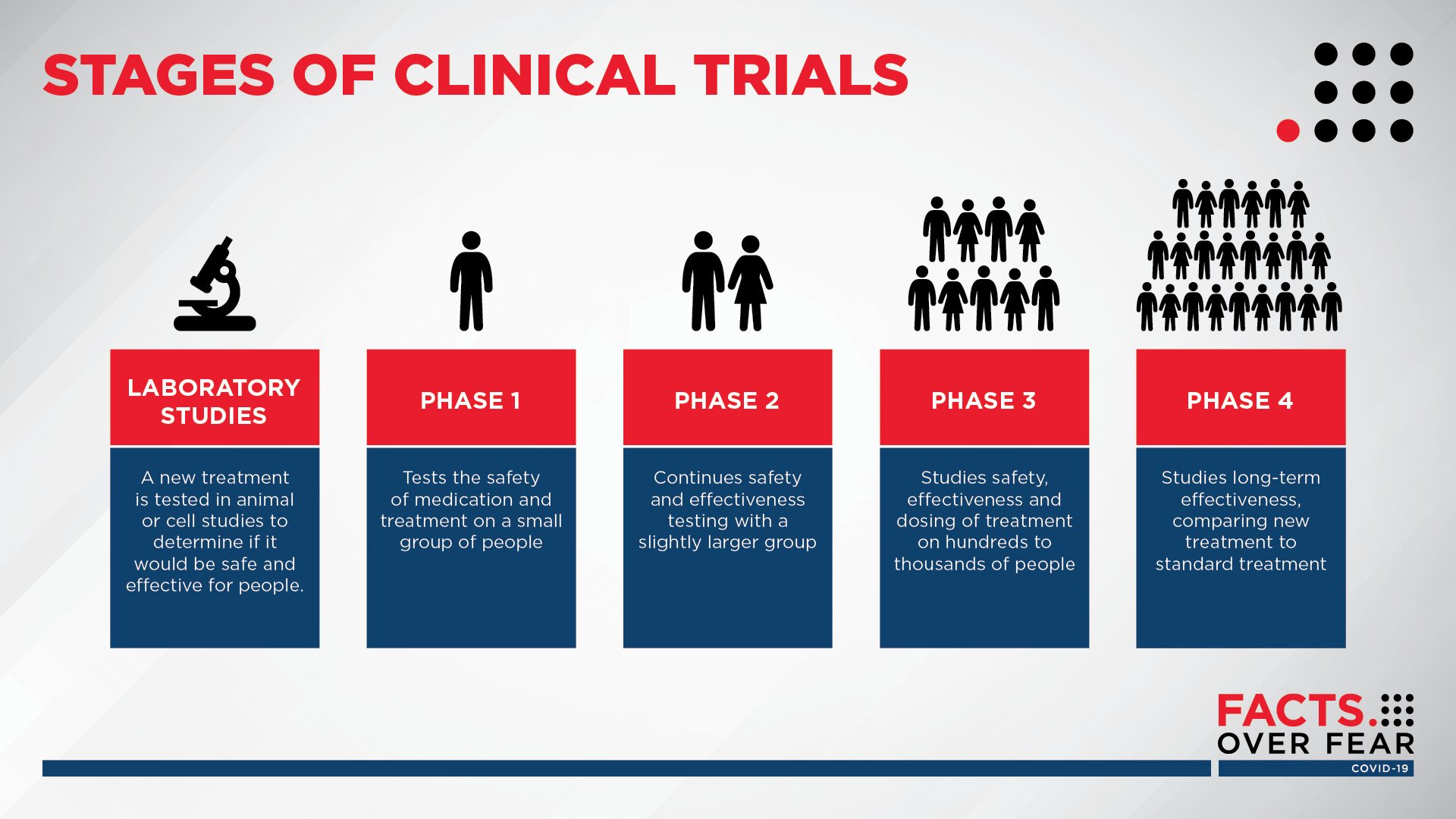
Large clinical trials are required to.
Vaccines in clinical trials. Because people with compromised immune systems were largely excluded from the Covid-19 vaccine trials in the US. And globally its unknown how much protection they get from vaccination. NEW YORK WABC – A new clinical trial is underway in Arizona for another COVID vaccine trying to enter the US.
Marketplace - this one focusing on adolescents. While most vaccine studies are relatively long clinical trials often around six to 12 months safety and efficacy can often be established earlier than the end of the. Pfizer Trial NCT04368728.
12 and older Number of people all ages. 46663 Clinical trial phase. What the trial is looking at.
The trial is looking to determine the safety and tolerability of this vaccineResearchers are also analyzing the vaccines ability to trigger an immune response and how well it works at preventing COVID-19. Cuba rolls out two Covid-19 vaccines still in clinical trials. Faced with a surge in coronavirus cases Cuba this week started immunizing members of the public using two locally-produced vaccines.
The Oxford-AstraZeneca and Pfizer BioNTech vaccines which are available in the UK went through clinical trials here to prove their safety and efficacy. During the trials half of. Clinical trials determine if COVID-19 vaccines are safe and effective.
Vaccines are complex biological products. There are strict evidence requirements that need to be met before they can be authorized for use in healthy people. Large clinical trials are required to.
Despite having undergone clinical trials vaccines made available under an EUA have not been scrutinized by the the same type of review as an FDA-approved or cleared product the FDA says. If a trial participant finds out they received the vaccine candidate in the clinical trial and not a placebo the NIHR website currently states. If you have received the full trial dose or.
This study is a randomized double-blinded and placebo controlled phase III clinical trial of the SARS-CoV-2 inactivated vaccine manufactured by Sinovac Research Development Co Ltd. The purpose of this study is to evaluate the efficacy safety and immunogenicity of the experimental vaccine in healthy adults aged 1859 Years. A Randomized Observer-Blind Dose-escalation Phase III Clinical Trial of Ad5-nCoV Vaccine in.
Researchers are currently testing 89 vaccines in clinical trials on humans and 27 have reached the final stages of testing. At least 77 preclinical vaccines are under active investigation in. MRNA Vaccines Work in Clinical Trials And in the Real World By Josh Bloom March 25 2021 There has been no shortage of COVID-19 vaccine doubters.
One of the infinite number of criticisms of the mRNA vaccines is that clinical trial data is somehow unreliable or that the vaccine wont work in the real world. Coronavirus vaccines have generally produced mild short-lived side effects both in and out of clinical trials. But emerging evidence suggests that Pfizers side effects may be.
New Delhi India May 14 ANI. A plea has been filed in the Supreme Court seeking transparency in Covid-19 vaccine clinical trial and post-vaccination data. Nearly two dozen coronavirus vaccines are in clinical trials while another 140 are in early development.
But some scientists are calling for volunteers to be exposed to the virus to accelerate. Clinical trials are research studies that determine whether medical products like medicines vaccines or devices are safe and effective. It important for participants in clinical trials to.