
Modified FOLFIRINOX has promising activity in nonmetastatic disease. To directly compare the efficacy and toxicity of standard-dose FOLFIRINOX sFOLFIRINOX and modified-dose FOLFIRINOX mFOLFIRINOX 75 of standard-dose for pancreatic cancer.
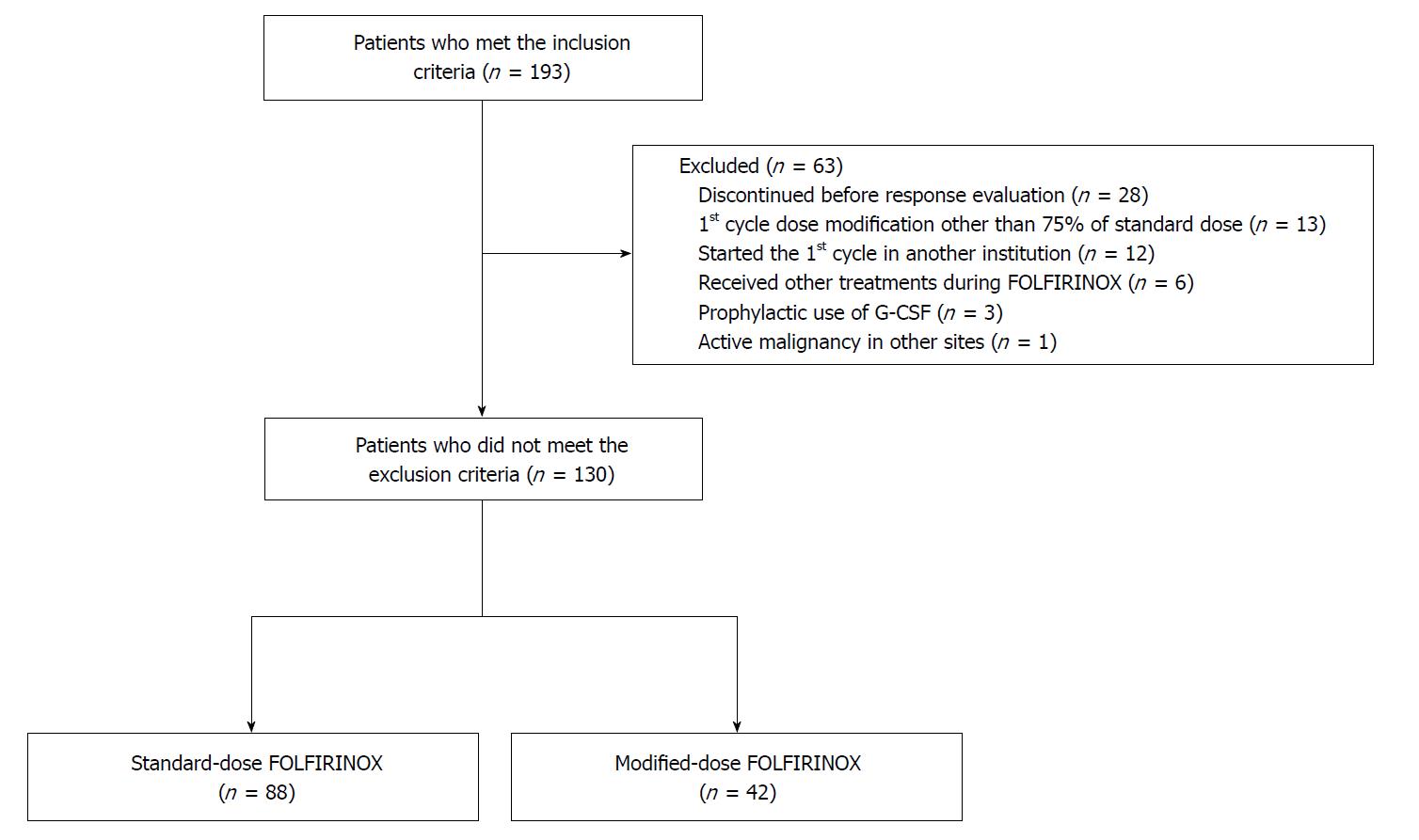
We conducted an institutional study to compare the clinical and pathological efficacy between the neoadjuvant therapy NATmodified FOLFIRINOX mFOLF vs nanoparticle albuminbound paclitaxel plus gemcitabine nabPG for borderline resectable pancreatic cancer BRPC and locally advanced pancreatic cancer LAPC patients who completed resection.
Modified folfirinox vs folfirinox. ASCO Guideline Update. Modified FOLFIRINOX Is Preferred as Adjuvant Chemotherapy in Patients With Resected Pancreatic Adenocarcinoma. In conclusion modified FOLFIRINOX could provide comparative survival benefits with fewer adverse events compared to the conventional dosage.
To directly compare the efficacy and toxicity of standard-dose FOLFIRINOX sFOLFIRINOX and modified-dose FOLFIRINOX mFOLFIRINOX 75 of standard-dose for pancreatic cancer. The overall survival rate at 3 years was 634 in the modified-FOLFIRINOX group and 486 in the gemcitabine group. Adverse events of grade 3 or 4 occurred in 759 of the patients in the modified.
The efficacy of this modified FOLFIRINOX was comparable to that of the original regimen but with a notably lower rate of adverse events. This modified FOLFIRINOX regimen might be a promising first-line chemotherapy option for Chinese patients with metastatic PC. Modified FOLFIRINOX has an improved safety profile with maintained efficacy in metastatic PC.
Modified FOLFIRINOX has promising activity in nonmetastatic disease. Modified FOLFIRINOX has promising activity in nonmetastatic disease. At baseline of second-line treatment there was no difference in patients characteristics between mFFX group and GnP group.
No significant difference in the response rate mFFX 166 vs. GnP 105 P 063 or the disease control rate mFFX 50 vs. GnP 64 P 082 was seen between.
At 3 years the modified folfirinox group had a DFS rate of 397 95 CI 328-466 vs. 214 95 CI 158-275 in the gemcitabine group. Patients received a modified dose of FOLFIRINOX regimen.
Oxaliplatin 65 mgm 2 leucovorin 400 mgm 2 irinotecan 150 mgm 2 and continuous infusion of fluorouracil 2400 mgm 2 over 46 h. FOLFIRINOX was discontinued until patients presented disease. ヵ月 vs FOLFIRINOX療法 64ヵ月 ハザード.
比 047 p. は好中球数減少210 vs 457 p. 発熱性好中球減少症12 vs 54 p003.
This study aimed to compare mFFX with full-dose FOLFIRINOX fFFX in patients with advanced pancreatic cancer APC. We reviewed 85 patients with APC who received mFFX no bolus fluorouracil and irinotecan 150 mg per square or fFFX as first-line chemotherapy between January 2014 and December 2016. MFFX has been used since January 2016 on the basis of results of a Japanese.
We conducted an institutional study to compare the clinical and pathological efficacy between the neoadjuvant therapy NAT-modified FOLFIRINOX mFOLF vs nanoparticle albumin-bound paclitaxel plus gemcitabine nab-PG for borderline resectable pancreatic cancer BRPC and locally. In conclusion in this single-center retrospective analysis of 24 patients with unresectable pancreatic cancer age 75 and older treated with modified dosing FOLFIRINOX the safety of this regimen appeared to be similar to what was reported by Conroy et al. In the original 2011 study describing the efficacy of FOLFIRINOX with comparable rates of hematologic toxicity.
The objective response rate was 371 in the FOLFOXIRI group vs 478 in the FOLFIRINOX group P 0187. Grade 34 toxicities occurred in 287 of patients in the FOLFOXIRI cohort vs 195 in the FOLFIRINOX cohort P 0079. FOLFOXIRI was associated with a higher incidence of grade 34 digestive adverse events.
FOLFIRINOX is a chemotherapy regimen for treatment of advanced pancreatic cancer. It is made up of the following four drugs. FOL folinic acid leucovorin a vitamin B derivative that enhances the effects of 5-fluorouracil 5-FU.
F fluorouracil 5-FU a pyrimidine analog and antimetabolite which incorporates into the DNA molecule and stops. We conducted an institutional study to compare the clinical and pathological efficacy between the neoadjuvant therapy NATmodified FOLFIRINOX mFOLF vs nanoparticle albuminbound paclitaxel plus gemcitabine nabPG for borderline resectable pancreatic cancer BRPC and locally advanced pancreatic cancer LAPC patients who completed resection. Pancreas adjuvant FOLFIRINOX modified fluorouracil leucovorin irinotecan oxaliplatin There are multiple FOLFIRINOX modified protocols.
This is the metastatic pancreatic cancer protocol and should not be used in the adjuvant setting. We conducted an institutional study to compare the clinical and pathological efficacy between the neoadjuvant therapy NATmodified FOLFIRINOX mFOLF vs nanoparticle albuminbound paclitaxel plus gemcitabine nabPG for borderline resectable pancreatic cancer BRPC and locally advanced pancreatic cancer LAPC patients who completed resection. No treatment-related death was observed.
The median overall survival and median progression-free survival are 103 months and 70 months respectively. In conclusion modified-FOLFIRINOX had significantly improved tolerance with similar efficacy to FOLFIRINOX. These findings may provide evidence for the use of FOLFIRINOX in Chinese patients with MPC.