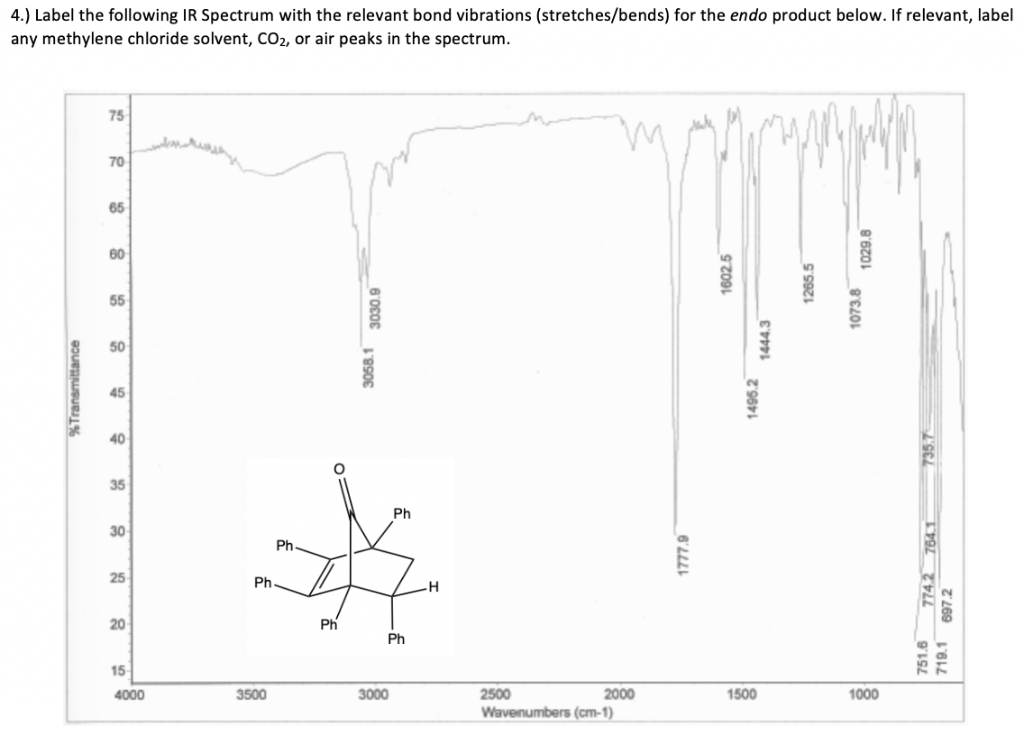
Methylene chloride also is used in the following industrial settings. The above IR spectrum is for methylene chloride and there are many peaks which correspond to certain vibrations in methylene chloride.
It is also used as a degreaser painter stripper and in the food industry to decaffeinate tea and coffee.
Ir of methylene chloride. Vibrational model of methylene chloride. The above IR spectrum is for methylene chloride and there are many peaks which correspond to certain vibrations in methylene chloride. The peaks around 3100 1cm is due to the asymmetric stretching between the C-H atoms.
The large peak around 1250 1cm is due to the H-C-H out of plane wagging. The IR-spectrum can be divided into five ranges major ranges of interest for an organic chemist. From 2700-4000 cm-1 E-H-stretching.
EB C N O In this range typically E-H-stretching modes are observed. The C-H-stretching modes can be found between 2850 and 3300 cm-1 depending on the hydrization. A common synonym for methylene chloride is dichloromethane.
14 Methylene chloride is a colorless liquid with a sweetish odor. 16 The chemical formula for methylene chloride is CH 2 Cl 2 and the molecular weight is 8493 gmol. 1 The vapor pressure for methylene chloride is 349 mm Hg at 20 C and it has an octanolwater coefficient log K ow.
Methylene chloride an organic compound is also known as DCM or dichloromethane. It is a volatile liquid with a sweet scent and mostly used as a solvent. Although it isnt miscible in water it is miscible with several organic solvents.
It is also used as a degreaser painter stripper and in the food industry to decaffeinate tea and coffee. Staff contact for methylene chloride. Ingrid Feustel Feustelingridepagov 202-564-3199.
Public dockets for methylene chloride. Below is information on EPA actions to manage risks from methylene chloride and protect public health. Methylene chloride also known as dichloromethane and DCM is a volatile.
If you need to find the frequency of a material go to the IR table by compound. Absorption cm -1 Appearance. Methylene Chloride Methylene chloride most often affects the central nervous system the brain causing headaches nausea dizziness clumsiness drowsiness and other effects like those of drinking alcohol.
At very high levels it can cause unconsciousness and death. Methylene chloride causes cancer in animals and is regulated as a cancer-causing substance in the workplace. Because it forms.
Dichloromethane CH2Cl2 CID 6344 - structure chemical names physical and chemical properties classification patents literature biological activities safety. Can irritate the nose and throat. Can harm the nervous system.
Symptoms may include headache nausea dizziness drowsiness and confusion. Methylene chloride form carbon monoxide in the body. METHYLENE CHLORIDE Methyleni chloridum CH2Cl2 Mr 849 DEFINITION Methylene chloride is dichloromethane.
It may contain not more than 20 per centVV of ethanol andor not more than 003 per cent VV of 2-methylbut-2-ene as stabiliser. CHARACTERS A clear colourless volatile liquid sparingly soluble in water miscible with alcohol. FROM SOURCES OF METHYLENE CHLORIDE Final Report Prepared for.
Dallas Safriet Emission Inventory Branch U. Environmental Protection Agency Research Triangle Park North Carolina 27711 Prepared by. Radian Corporation Post Office Box 13000 Research Triangle Park North Carolina 27709 April 22 1993.
This report has been reviewed by the Office Of Air Quality Planning And Standards. Heavy metals Method I 231 Evaporate 15 mL 20 g in a glass evaporating dish on a steam bath to dryness. Cool add 2 mL of hydrochloric acid and slowly evaporate again on a steam bath to dryness.
Dissolve the residue in 1 mL of 1 N acetic acid add 24 mL of water and mix. The limit is 1 ppm. Empirical Formula Hill Notation.
CH 2 Cl 2. Methylene chloride is a solvent which is used in many different types of work activities such as paint stripping polyurethane foam manufacturing cleaning and degreasing. Employees exposed to methylene chloride are at increased risk of developing cancer adverse effects on the heart central nervous system and liver and skin or eye irritation.
Exposure may occur through inhalation by. Methylene chloride also called dichloromethane is a volatile colorless liquid with a chloroform-like odor. Methylene chloride is used in various industrial processes in many different industries including paint stripping pharmaceutical manufacturing paint remover manufacturing and metal cleaning and degreasing.
The most common means of exposure to methylene chloride is inhalation and skin exposure. OSHA considers methylene chloride. Methylene chloride is most prominently used industrially in the production of paint strippers pharmaceuticals and process solvents.
Methylene chloride also is used in the following industrial settings. Food and Beverage Manufacturing. Methylene chloride is used as an extraction solvent in the food and beverage manufacturing industry.
For example methylene chloride can be used to remove.