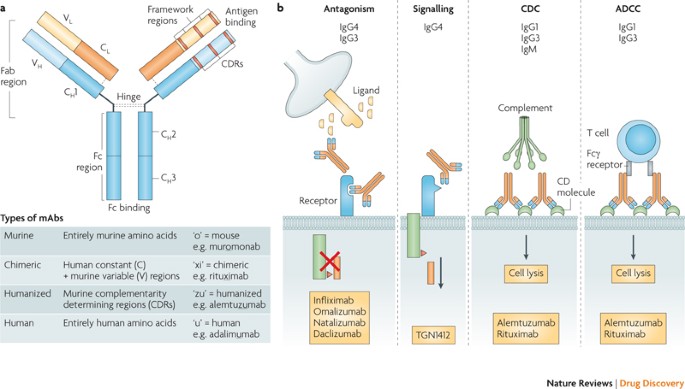
In addition there are numerous adverse effects of mAbs that are related to their specific targets including infections and cancer autoimmune disease and organ-specific adverse events such as cardiotoxicity. Patients must be older than 12 years have a positive skin test to a perennial aeroallergen eg dust mites cats dogs and mold and be symptomatic with inhaled corticosteroids.
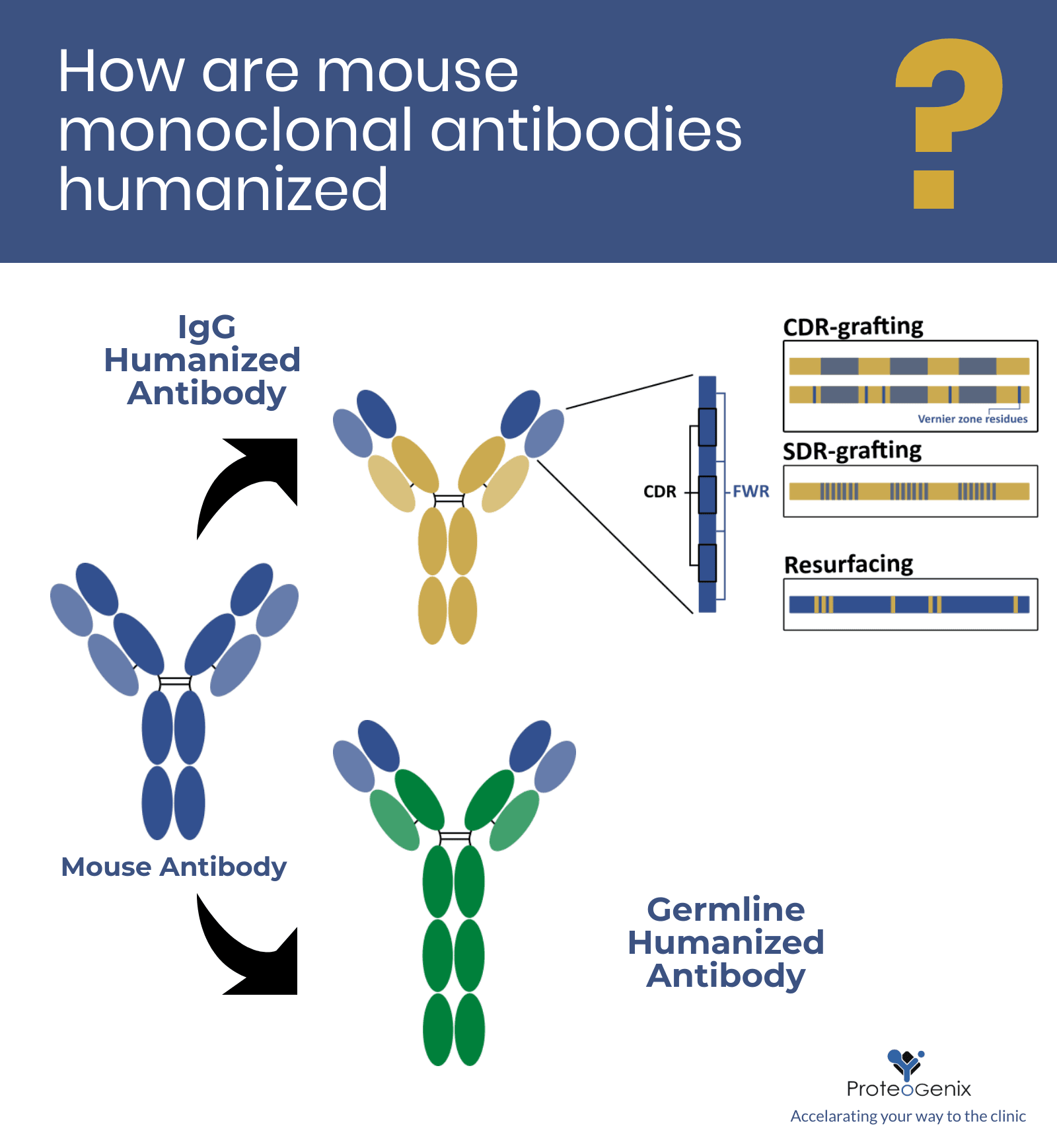
Bevacizumab is a humanized immunoglobulin G monoclonal antibody that binds to VEGF with high specificity thereby blocking VEGF-mediated signalling pathways and thus angiogenesis.
Humanized monoclonal antibody side effects. Possible side effects can include. Fever Chills Weakness Headache Nausea Vomiting Diarrhea Low blood pressure Rashes. However administration of mAbs carries the risk of immune reactions such as acute anaphylaxis serum sickness and the generation of antibodies.
In addition there are numerous adverse effects of mAbs that are related to their specific targets including infections and cancer autoimmune disease and organ-specific adverse events such as cardiotoxicity. In March 2006 a life-threatening cytokine release. Common side effects include mild to moderate chills fever nausea and respiratory symptoms with initial dose.
50-52 Abdominal pain diarrhea vomiting headaches and rash also occur and increase in frequency when trastuzumab is used in combination with chemotherapy. Other side effects of monoclonal antibodies include. Shortness of breath Peripheral edema Headache Fever Muscle aches and pain Decreased appetite Increased triglyceride levels Insomnia Abdominal pain Back pain Dizziness.
During the therapy period one patient had parasitosis giardiasis one patient had severe side effects one patient had dyspnea two hours after the injection and one patient had a dyspnea attack 2 hours after the injection. The changes in MPV levels were not statistically significant. There was also a significant decrease in IgE levels after the treatment.
With improved therapeutic strategies due to these agents however there are also various sometimes unexpected side effects. Most of the monoclonal antibodies used in oncology share a risk of infusion-related manifestations including the possibility of anaphylaxis. These reactions usually appear early on during the first administration.
Hematological toxicity is also frequent especially if the antibodies. Numerous adverse effects of mAbs that are related to their specific targets including infections and cancer autoimmune disease and organ-specific adverse events such as cardiotoxicity. Monoclonal antibodies mAbs are now established as targeted therapies for malignancies transplant rejection autoimmune and infectious diseases as.
However major causes of concern while using these drugs are risk of susceptibility to infection and development of anti-drug antibodies which will affect the pharmacokinetic properties efficacy and safety profile of the drug. Itolizumab a humanized anti-CD6 monoclonal antibody is a new molecule that acts by immunomodulating the CD6 molecule. CD6 is a co-stimulatory molecule required for.
A 41-year-old woman with active seropositive erosive rheumatoid arthritis was treated with the humanized monoclonal antibody Campath 1H. She had not responded or developed side effects to myocrisin sulfasalazine and penicillamine and had not responded to inpatient bedrest and physiotherapy. There was a rapid clinical improvement within 24 hours of infusion which was.
Omalizumab Xolair is a recombinant humanized monoclonal antibody that blocks the high-affinity Fc receptor of immunoglobulin E IgE. It is used to treat patients with moderate-persistent to severe-persistent asthma. Patients must be older than 12 years have a positive skin test to a perennial aeroallergen eg dust mites cats dogs and mold and be symptomatic with inhaled corticosteroids.
Omalizumab has a low incidence of side effects. The binding specificity of the murine OKT3 has been transferred into a human antibody framework to reduce its immunogenicity. This humanized anti-CD3 mAb gOKT3-5 was previously shown to retain in vitro all the properties of native OKT3 including T cell activation which has been correlated in vivo with the severe side effects observed in transplant recipients after the first administration of the mAb.
Bevacizumab is a humanized immunoglobulin G monoclonal antibody that binds to VEGF with high specificity thereby blocking VEGF-mediated signalling pathways and thus angiogenesis. Clinical trials have shown that bevacizumab is effective in prolonging survival in patients with metastatic colorectal cancer CRC when combined with standard chemotherapy. Consequently bevacizumab has been.
In general the more common side effects caused by monoclonal antibody drugs include. Allergic reactions such as hives or itching Flu-like signs and symptoms including chills fatigue fever and muscle aches and pains. A monoclonal antibody is an antibody made by cloning a unique white blood cell.
All subsequent antibodies derived this way trace back to a unique parent cell. Monoclonal antibodies can have monovalent affinity binding only to the same epitope. In contrast polyclonal antibodies bind to multiple epitopes and are usually made by several different antibody secreting plasma cell lineages.
Toxicity included thrombocytopenia in four and lymphocytopenia in seven patients. Although the antibody is humanized the nonhematological toxicity was substantial and probably due to a cytokine release syndrome. Prophylactic treatment of the side effects is strongly recommended for patients treated either with CP-1H alone.
A recombinant humanized anti-cocaine monoclonal antibody mAb h2E2 is at an advanced stage of pre-clinical development as an immunotherapy for cocaine abuse. It is hypothesized that h2E2 binds to and sequesters cocaine in the blood. A three-compartment model of the effects of h2E2 on cocaines distribution was constructed.
The model assumes that h2E2 binds to. The most common side effects include fever and rash. Palivizumab is a humanized monoclonal antibody IgG directed against an epitope in the A antigenic site of the F protein of RSV.
In two phase III clinical trials in the pediatric population palivizumab reduced the.