Organocopper compounds are diverse in structure and reactivity but organocopper compounds are largely limited in oxidation states to copperI sometimes denoted Cu As a d 10 metal center it is related to Ni0 but owing to its higher oxidation state it engages in less pi-backbonding. Bonding The atoms in covalent network compounds are held together by covalent bonds.

The geometric structure and bonding pattern of selected copper I iodide clusters Cu 4 I j 4j 2 j 8 were understood by density functional theory DFT.
Copper structure and bonding. The coordination chemistry of copperI carbonyls and cyano complexes is reviewed. Primary attention is focused on structural chemistry including coordination behavior bridging modes network formation flexible coordination number and reversible ligand binding. Additional coverage of infrared spectroscopy and orbital interactions in carbonyl and cyanide bonding and the.
In this work we analyze CO binding on small neutral copper clusters Cun n 19. Molecular structures and reactivity descriptors of copper clusters are computed and discussed. The results show that the condensed Fukui functions and the frontier molecular orbital theory are useful tools to predict the selectivity of CO adsorption on these small clusters.
To get further insight into the CO binding to. Copper nitrenes are of interest as intermediates in the catalytic aziridination of olefins and the amination of CH bonds. However despite advances in the isolation and study of late-transition-metal multiply bonded complexes a bona fide structurally characterized example of a terminal copper nitrene has to our knowledge not been reported.
Copper nitrenes are of interest as intermediates in the catalytic aziridination of olefins and the amination of C-H bonds. However despite advances in the isolation and study of late-transition-metal multiply bonded complexes a bona fide structurally characterized example of a terminal copper nitrene has to our knowledge not been reported. Structure and Bonding in CopperI Carbonyl and Cyanide Complexes.
Pike Department of Chemistry College of William and Mary Williamsburg VA 23187-8795. RECEIVED DATE to be automatically inserted after your manuscript is accepted if required according to the journal that you are submitting your paper to. The geometric structure and bonding pattern of selected copper I iodide clusters Cu 4 I j 4j 2 j 8 were understood by density functional theory DFT.
The global minimum structure of each cluster was predicted from first principles and important geometric and electronic properties were evaluated and summarized. Both Wang and Illas notice that the bonding in copper I hydroxide is characterized by a subtle interplay between covalent and ionic contributions. This aspect and in particular the contribution of electrostatic interactions will be central in our analysis of the bonding in copper I chalcogenides.
Chiral and achiral copperii complexes. Structure bonding and biological activities Assila Maatar Ben Salah a Nadhem Sayari b Houcine Naïli a and Alexander. Norquist c Author affiliations Corresponding authors a Laboratoire Physicochimie de lEtat Solide Département de Chimie Faculté des Sciences de Sfax Université de Sfax 3000 Sfax Tunisia E-mail.
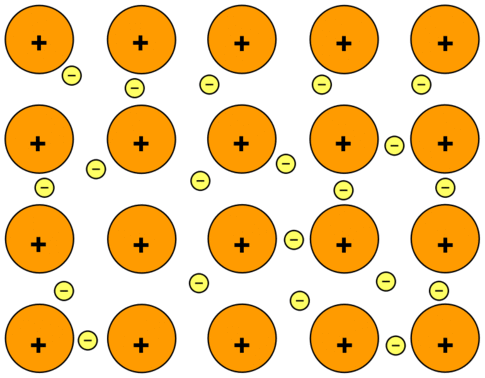
Copper is a good material for making a kettle because. It has a high melting point it is a very good conductor of heat. A Explain why copper like many other metals has a high melting point.
Your answer should describe the structure and bonding of a metal. The structure of a solid metal consists of closely packed metal ions arranged in a regular way to form a metallic lattice structure. Metallic bonding is the strong electrostatic force of.
Bonding The atoms in covalent network compounds are held together by covalent bonds. Covalent bonds are strong. Structure Diamond and silica atoms are covalently bonded in regular 3 dimensional networks each atom is bonded to 4 other atoms.
Graphite atoms are covalently bonded in. Copper nitrenes are of interest as intermediates in the catalytic aziridination of olefins and the amination of C-H bonds. However despite advances in the isolation and study of late-transition-metal multiply bonded complexes a bona fide structurally characterized example of a terminal copper nitrene has to our knowledge not been reported.
Organocopper compounds are diverse in structure and reactivity but organocopper compounds are largely limited in oxidation states to copperI sometimes denoted Cu As a d 10 metal center it is related to Ni0 but owing to its higher oxidation state it engages in less pi-backbonding. Organic derivatives of CuII and CuIII are invoked as intermediates but rarely. The coordination chemistry of copperI carbonyls and cyano complexes is reviewed.
Primary attention is focused on structural chemistry including coordination behavior bridging modes network formation flexible coordination number and reversible ligand binding. Additional coverage of infrared spectroscopy and orbital interactions in carbonyl and cyanide bonding and the photophysical. A calculation is made of the electronic structure of the row of crystals with copperoxygen bonding.
It is shown that the covalence effects are essential for copper oxide compounds containing copper atoms in oxydation states Cu II and Cu III LUCCNDO calculations of La 2 CuO 4 and YBa 2 Cu 3 O x x 67 crystals are made. They have relatively large atoms meaning that the nuclei are some distance from the delocalised electrons which also weakens the bond. The delocalised electrons are free to move throughout the structure in 3-dimensions.
They can cross grain boundaries. Even though the pattern may be disrupted at the boundary as long as atoms are touching each other the metallic bond. They are good conductors of thermal energy because their delocalised electrons transfer energy.
They have high melting points and boiling points because the metallic bonding in the giant. Structure Bonding and Catecholase Mechanism of Copper Bispidine Complexes Inorganic Chemistry. Oxygen activation by copperI complexes with tetra- or pentadentate mono- or dinucleating bispidine ligands is known to lead to unusually stable end-on-bispidineCu2O22 complexes bispidines are.
Findings The problemschallenges such as wire open and short tail defects poor bondability for stitchwedge bonds oxidation of Cu wire strain-hardening effects and stiff wire on weak support.